There are no head-to-head studies comparing the safety or efficacy between XTAMPZA ER and OxyContin.
Chewing or crushing some pain medications can result in a dangerous spike of medication levels in your blood.
XTAMPZA ER offers the ability to open the capsule and sprinkle the medicine if you dislike or have trouble swallowing capsules, without resulting in a dangerous spike in medication when used as prescribed.
XTAMPZA ER MUST be taken with food to help your body absorb the medicine.

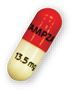






 10 mg
10 mg
 15 mg
15 mg
 20 mg
20 mg
 30 mg
30 mg
 40 mg
40 mg
 60 mg
60 mg
 80 mg
80 mg
Because the use of XTAMPZA ER exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death, assess each patient's risk prior to prescribing reassess all patients regularly for the development of these behaviors or conditions.
Serious, life-threatening, or fatal respiratory depression may occur with use of XTAMPZA ER, especially during initiation or following a dosage increase. To reduce the risk of respiratory depression, proper dosing and titration of XTAMPZA ER are essential.
Accidental ingestion of even one dose of XTAMPZA ER, especially by children, can result in a fatal overdose of oxycodone.
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of XTAMPZA ER and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate.
Advise pregnant women using opioids for an extended period of time of the risk of Neonatal Opioid Withdrawal Syndrome, which may be life-threatening if not recognized and treated. Ensure that management by neonatology experts will be available at delivery.
Healthcare providers are strongly encouraged to complete a REMS-compliant education program and to counsel patients and caregivers on serious risks, safe use, and the importance of reading the Medication Guide with each prescription.
The concomitant use of XTAMPZA ER with all cytochrome P450 3A4 inhibitors may result in an increase in oxycodone plasma concentrations, which could increase or prolong adverse drug effects and may cause potentially fatal respiratory depression. In addition, discontinuation of a concomitantly used cytochrome P450 3A4 inducer may result in an increase in oxycodone plasma concentration. Regularly evaluate patients receiving XTAMPZA ER and any CYP3A4 inhibitor or inducer.
These are not all the possible side effects of XTAMPZA ER. Call your healthcare provider for medical advice about side effects. You may report side effects to the FDA at 1‑800‑FDA‑1088. For more information, go to dailymed.nlm.nih.gov.
See full Prescribing Information, including Boxed Warning on Addiction, Abuse and Misuse and other serious risks, and the Medication Guide accompanying this piece or at XTAMPZAER.com/PI. Speak to your healthcare provider if you have questions about XTAMPZA ER.